Physical Revolution in Chemistry: the Core Ideas behind the Bohr Model of the Atom
DOI:
https://doi.org/10.15170/PAAA.2022.09.02.06.Keywords:
atomic theory, atomic spectra, nucleus, electron, quantum mechanicsAbstract
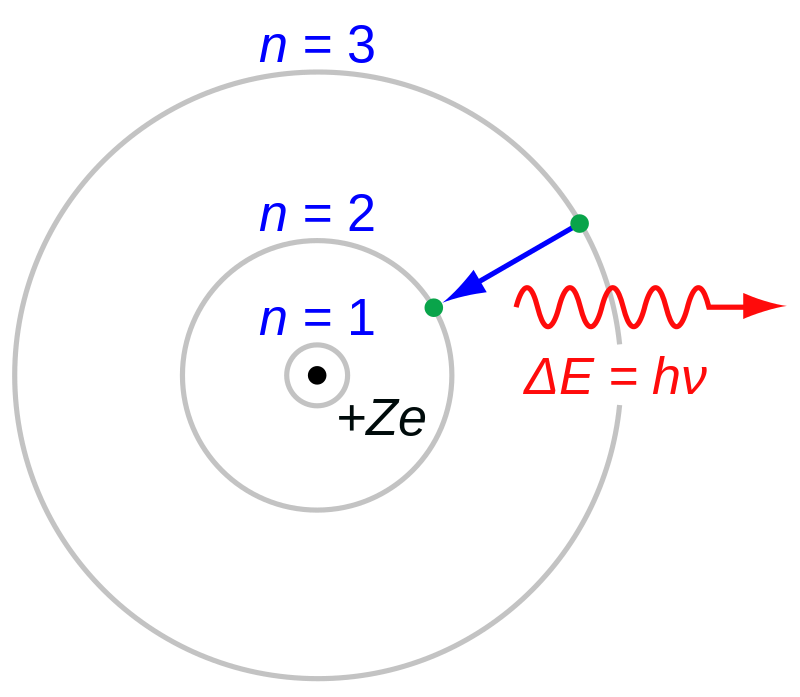
Niels Bohr published the fundamentals of the atom model later named after him in three scientific papers in 1913. This article explores the origins of the famous postulates in the model and also discusses how these were validated experimentally in later discoveries. The postulate that states the circular movement of the electron around the nucleus contradicted the Maxwell equations, which were well established in 1913 and required the emission of electromagnetic radiation in such cases. However, the assumed scenario followed directly from sound experimental findings. The truly novel element was the quantum postulate, which enabled a fully quantitative interpretation of the known atomic spectrum of the hydrogen atom. To establish the postulate, Bohr assumed a connection between the frequency of the circular motion of the electron and the frequency of the emitted electromagnetic radiation. At the time, the utility of this assumption was shown by the agreement of the conclusions drawn from it with experimental observations. The physical background of the quantum postulate was discovered later by seemingly remote findings: the wave-particle duality, the quantum mechanical uncertainty principle, and the experimental confirmation of the fact that the angular momentum of the photon is a constant that does not depend on its energy. In this sense, the postulates that were originally set up only to provide the framework for an atom model became the harbingers of later major developments in physics already given in the form of an exact mathematical equation.
Downloads
References
Balmer, Johann Jakob: Notiz über die Spectrallinien des Wasserstoffs. Annalen der Physik 261. (1885):5. 80–87. ǁ [DOI] https://doi.org/10.1002/andp.18852610506 ǁ [Google Scholar] https://tinyurl.com/57whwv3t
Brackett, Theodore: Visible and infra-red radiation of hydrogen. Astrophysical Journal 56. (1922):3. 154–161. ǁ [DOI] https://doi.org/10.1086/142697 ǁ [Google Scholar] https://tinyurl.com/y5kurcec
Bohr, Niels: On the constitution of atoms and molecules. Philosophical Magazine Series (6) 26. (1913):151. 1–25. ǁ [DOI] https://doi.org/10.1080/14786441308634955 ǁ [Google Scholar] https://tinyurl.com/2mnau2x8
Bohr, Niels: On the constitution of atoms and molecules. Part II. - Systems containing only a single nucleus. Philosophical Magazine Series (6) 26. (1913):153. 476–502. ǁ [DOI] https://doi.org/10.1080/14786441308634993
Bohr, Niels: On the constitution of atoms and molecules. Part III. - Systems containing several nuclei. Philosophical Magazine Series (6) 26. (1913):155. 857–875. ǁ [DOI] https://doi.org/10.1080/14786441308635031
Bohr, Niels: The spectra of helium and hydrogen. Nature 92. (1913):2295. 231–232. ǁ [DOI] https://doi.org/10.1038/092231d0 ǁ [Google Scholar] https://tinyurl.com/4tw3awve
Coulomb, Charles-Augustin: Premier mémoire sur l’électricité et le magnétisme. In: Histoire de l’Académie Royale des Sciences. Paris, 1785. 569–577.
Coulomb, Charles-Augustin: Second mémoire sur l’électricité et le magnétisme. In: Histoire de l’Académie Royale des Sciences. Paris, 1785. 578–611.
Coulomb, Charles-Augustin: Troisième mémoire sur l’électricité et le magnétisme. In: Histoire de l’Académie Royale des Sciences. Paris, 1785. 611–638.
Dalton, John: A new System of Chemical Philosophy. London, 1808. ǁ [DOI] https://doi.org/10.5479/sil.324338.39088000885681 ǁ [Google Scholar] https://tinyurl.com/2e52kdus
De Broglie, Louis: Waves and Quanta. Nature 112. (1923):2815. 540–540. ǁ [DOI] https://doi.org/10.1038/112540a0 ǁ [Google Scholar] https://tinyurl.com/mrye7fbk
Einstein, Albert: Über einen die Erzeugung und Verwandlung des Lichtes betreffenden heuristischen Gesichtspunkt. Annalen der Physik 322. (1905):1. 132–148. ǁ [DOI] https://doi.org/10.1002/andp.19053220607 ǁ [Google Scholar] https://tinyurl.com/mt9cmkuj
Einstein, Albert: Zur Theorie der Lichterzeugung und Lichtabsorption. Annalen der Physik 325. (1906):6. 199–206. ǁ [DOI] https://doi.org/10.1002/andp.19063250613 ǁ [Google Scholar] https://tinyurl.com/y4894pnb
Einstein, Albert: Die Plancksche Theorie der Strahlung und die Theorie der spezifischen Wärme. Annalen der Physik 327. (1907):1. 180–190. ǁ [DOI] https://doi.org/10.1002/andp.19063270110 ǁ [Google Scholar] https://tinyurl.com/3y7eh7yj
Heisenberg, Werner Karl: Über den anschaulichen Inhalt der quantentheoretischen Kinematik und Mechanik. Zeitschrift für Physik 43. (1927):3–4. 172–198. ǁ [DOI] https://doi.org/10.1007/BF01397280 ǁ [Google Scholar] https://tinyurl.com/57jj7bwh
Humphreys, Curtis Judson: The sixth series in the spectrum of atomic hydrogen. Journal of Research of the National Bureau of Standards 50. (1953):1. 1–6. ǁ [DOI] https://doi.org/10.6028/jres.050.001
Jammer, Max: The conceptual development of quantum mechanics. New York, 1966. ǁ [Google Scholar] https://tinyurl.com/mrympp46
Kirchhoff, Gustav Robert - Bunsen, Robert Wilhelm: Chemische Analyse durch Spectralbeobachtungen. Annalen der Physik 186. (1860):6. 161–189. ǁ [DOI] https://doi.org/10.1002/andp.18601860602 ǁ [Google Scholar] https://tinyurl.com/yebpe6m8
Kirchhoff, Gustav Robert - Bunsen, Robert Wilhelm: Chemical analysis by spectrum-observations. Philosophical Magazine Series (4) 20. (1860):131. 88–109. ǁ [DOI] https://doi.org/10.1080/14786446008642913 ǁ [Google Scholar] https://tinyurl.com/bd2a392w
Lyman, Theodore: The spectrum of hydrogen in the region of extremely short wave-length. Astrophysical Journal 23. (1906):3. 181–210. ǁ [DOI] https://doi.org/10.1086/141330 ǁ [Google Scholar] https://tinyurl.com/yn7n6smx
Maxwell, James Clerk: A dynamical theory of the electromagnetic field. Philosophical Transactions 155. (1865):1. 459–512. ǁ [DOI] https://doi.org/10.1098/rstl.1865.0008 ǁ [Google Scholar] https://tinyurl.com/3tkryape
Mengyelejev, Dmitrij Ivanovics: Cooтнoшeниe cвoйcтв c aтoмным вecoм элeмeнтoв. Журнал Русского Химического Общества 1. (1869):2–3., 60–77.
Mengyelejev, Dmitrij Ivanovics: Über die Beziehungen der Eigenschaften zu den Atomgewichten der Elemente. Zeitschrift für Chemie Neue Folge V. Band 12. (1869):1. 405–406.
Mérő László: Az ész segédigéi. Budapest, 2019.
Nicholson, John William: The spectrum of nebulium. Monthly Notices of the Royal Astronomical Society 72. (1911):1. 49–64. ǁ [DOI] https://doi.org/10.1093/mnras/72.1.49 ǁ [Google Scholar] https://tinyurl.com/3sjt74ac
Paschen, Friedrich: Zur Kenntnis ultraroter Linienspektra. I. (Normalwellenlängen bis 27000 Å.-E.). Annalen der Physik 332. (1908):13. 537–570. ǁ [DOI] https://doi.org/10.1002/andp.19083321303 ǁ [Google Scholar] https://tinyurl.com/3vnn5sjn
Pfund, August Herman: The emission of nitrogen and hydrogen in the infrared. Journal of the Optical Society of America 9. (1924):3. 193–196. ǁ [DOI] https://doi.org/10.1364/JOSA.9.000193 ǁ [Google Scholar] https://tinyurl.com/mckp8mzu
Planck, Max: Zur Theorie der Wärmestrahlung. Annalen der Physik 336. (1910):4. 758–768. ǁ [DOI] https://doi.org/10.1002/andp.19103360406 ǁ [Google Scholar] https://tinyurl.com/45dh3zh5
Planck, Max: Über die Begründung des Gesetzes der schwarzen Strahlung. Annalen der Physik 342. (1910):4. 642–656. ǁ [DOI] https://doi.org/10.1002/andp.19123420403 ǁ [Google Scholar] https://tinyurl.com/4ttj88s3
Planck, Max: Eine neue Strahlungshypothese. Verhandlungen der Deutschen Physikalischen Gesellschaft 13. (1911) 138–148.
Raman, Chandrasekhara Venkata - Bhagavantam, Suri: Spin of light quanta. Nature 128. (1931):3234. 727–727. ǁ [DOI] https://doi.org/10.1038/128727c0 ǁ [Google Scholar] https://tinyurl.com/ruy5cbdt
Russell, Alexander Stuart - Rossi, R.: An investigation of the spectrum of ionium. Proceedings of the Royal Society (London) A 87. (1912):598. 478–484. ǁ [DOI] https://doi.org/10.1098/rspa.1912.0100 ǁ [Google Scholar] https://tinyurl.com/3mbtezze
Rutherford, Ernest: The scattering of α and β particles by matter and the structure of the atom. Philosophical Magazine Series (6) 21. (1911):125. 669–688. ǁ [DOI] https://doi.org/10.1080/14786440508637080 ǁ [Google Scholar] https://tinyurl.com/mvsytzne
Rydberg, Johannes Robert: Researches sur la constitution des spectres d’émission des éléments chimiques. Kongliga Svenska Vetenskaps-Akademiens Handlingar 2nd series 23. (1890):11. 1–177. ǁ [Google Scholar] https://tinyurl.com/yc7untpw
Rydberg, Johannes Robert: On the structure of the line-spectra of the chemical elements. Philosophical Magazine Series (5) 29. (1890):179. 331–337. ǁ [DOI] https://doi.org/10.1080/14786449008619945 ǁ [Google Scholar] https://tinyurl.com/4sf8ntmj
Simonyi Károly: A fizika kultúrtörténete. Ötödik kiadás. Budapest, 2011. ǁ [Google Scholar] https://tinyurl.com/46maztdj
Tank, Franz: Über Serienspektren nach dem Bohrschen Modell. Annalen der Physik 364. (1919):12. 293–331. ǁ [DOI] https://doi.org/10.1002/andp.19193641202 ǁ [Google Scholar] https://tinyurl.com/52djfzrd
Thomson, Joseph John: Cathode rays. Philosophical Magazine Series (5) 44. (1897):269. 293–316. ǁ [DOI] https://doi.org/10.1080/14786449708621070 ǁ [Google Scholar] https://tinyurl.com/5y2289u9
Veszprémi Tamás: Általános kémia. Második kiadás. Budapest, 2015.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Per Aspera ad Astra

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.










